Introduction:
Many marrow failure conditions are associated with myeloid malignancy predisposition and a subset carry an increased risk of both myeloid and lymphoid malignancies. The increased risk of myeloid malignancy is known in Shwachman-Diamond syndrome (SDS), however, the risk of lymphoid malignancy in SDS has not been previously reported. Through the North American SDS Registry (SDSR), we identified patients with SDS diagnosed with lymphoma. We report the clinical features and outcomes in these patients as well as the incidence rates of lymphoma, MDS, and AML in this cohort.
Methods:
Patients with biallelic SBDS mutations enrolled on the SDSR were included for analysis. Age- and sex-specific incidence rates from the SEER Program (2016-2020) were multiplied by person-years of observation to obtain expected numbers of events. The crude rate per 100,000 (100,000*observed number of malignancies/person-years of follow-up) and the standardized incidence ratio (SIR) were calculated.
Results:
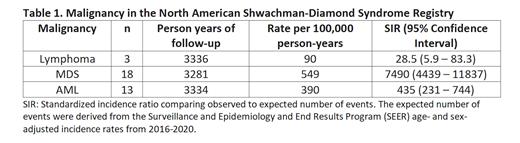
Out of a total of 217 patients with SDS with biallelic SBDS mutations in the SDSR, we identified 3 subjects who developed lymphoma.
Case 1: A male patient with biallelic SBDS mutations (c.258 + 2T>C; c.653G>A, p.Arg218Gln) presented with dyspnea and was diagnosed with primary mediastinal B-cell lymphoma at age 16 years. Bone marrow exam was negative for lymphoma but revealed a new del20q clone. He received 6 cycles of R-CHOP. His course was complicated by febrile neutropenia, pleural effusion, deep vein thrombosis, and decreased cardiac ejection fraction requiring subsequent omission of doxorubicin and carvedilol initiation. He is in remission >3 years from diagnosis.
Case 2: A male patient with biallelic SBDS mutations (c.258 + 2T>C; c.183_184TA>CT, p.Lys62*) was diagnosed at age 23 years with diffuse large B-cell lymphoma (DLBCL) of the distal stomach and duodenal bulb. Bone marrow exam was negative for malignancy but demonstrated an abnormal karyotype: 46,XY,t(11;16)(q23;q24), del(20)(q11.2q13.3)[9]/46,XY[11]. He received 5 cycles of R-CHOP therapy, which was complicated by febrile neutropenia and S. aureus sepsis. Four years later, he was diagnosed with relapsed DLBCL of the distal ileum and concurrent AML with 5q deletion. He achieved complete remission of both malignancies after 2 cycles of cyclophosphamide, vincristine, prednisone, obinutuzumab, azacitidine, and venetoclax. Therapy was complicated by B. cereus bacteremia with brain abscesses and endocarditis and C. difficile infection. He underwent a matched unrelated donor hematopoietic stem cell transplant and is in remission >180 days from transplant.
Case 3: A male patient with biallelic SBDS mutations (c.258 + 2T>C; c.183_184TA>CT, p.Lys62*) was diagnosed with Stage IVB classical Hodgkin lymphoma at age 12 years after presenting with mediastinal syndrome. Bone marrow biopsy was negative for findings suggestive of MDS or malignancy, however, the initial PET-CT was consistent with medullary involvement. He initiated therapy with steroids, doxorubicin, vinblastine, dacarbazine, and brentuximab vedotin and received focal radiation. His course was complicated by recurrent febrile neutropenia, C. difficile colitis, and cellulitis. Due to infectious complications and delayed count recovery after 2 cycles, despite chemotherapy dose reductions, he was transitioned to brentuximab and nivolumab. He demonstrated complete response by Deauville criteria prior to cycle 5 of brentuximab and nivolumab.
Among 217 patients with biallelic SBDS mutations in the SDSR, the median age was 12.8 years (range: 0.3 - 52.8). The observed number, rates, and SIR for lymphoma, MDS, and AML are given in Table 1. A high SIR indicates a higher incidence in the SDSR relative to the SEER population in 2016-2020. The observed rate of lymphoma was 28.5 times higher in SDS than the expected rate in the general population.
Conclusions: The incidence of lymphoma among patients with SDS is increased relative to the general population but is lower than the incidence of MDS or AML. Increased toxicity with standard therapies was observed in these patients. Given the increased risk of MDS/AML in SDS, which may be further increased by antecedent chemotherapy, non-genotoxic therapies should be investigated for lymphoma in SDS. Our results show that SDS should be added to the list of marrow failure conditions predisposing to both myeloid and lymphoid malignancies.
Disclosures
Farrar:Novartis: Research Funding. Myers:Incyte: Other: Clinical trial funding; Elixirgen Therapeutics: Other: Industry sponsored clinical trial . Shimamura:X4 Pharmaceuticals; Novartis: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal